GE23077 A1/B1
Product Code:
AG-CN2-0305
AG-CN2-0305
Host Type:
Bacteria
Bacteria
Regulatory Status:
RUO
RUO
Shipping:
AMBIENT
AMBIENT
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CN2-0305-M001 | 1 mg | £90.00 |
Quantity:
| AG-CN2-0305-M005 | 5 mg | £300.00 |
Quantity:
| AG-CN2-0305-M025 | 25 mg | £880.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
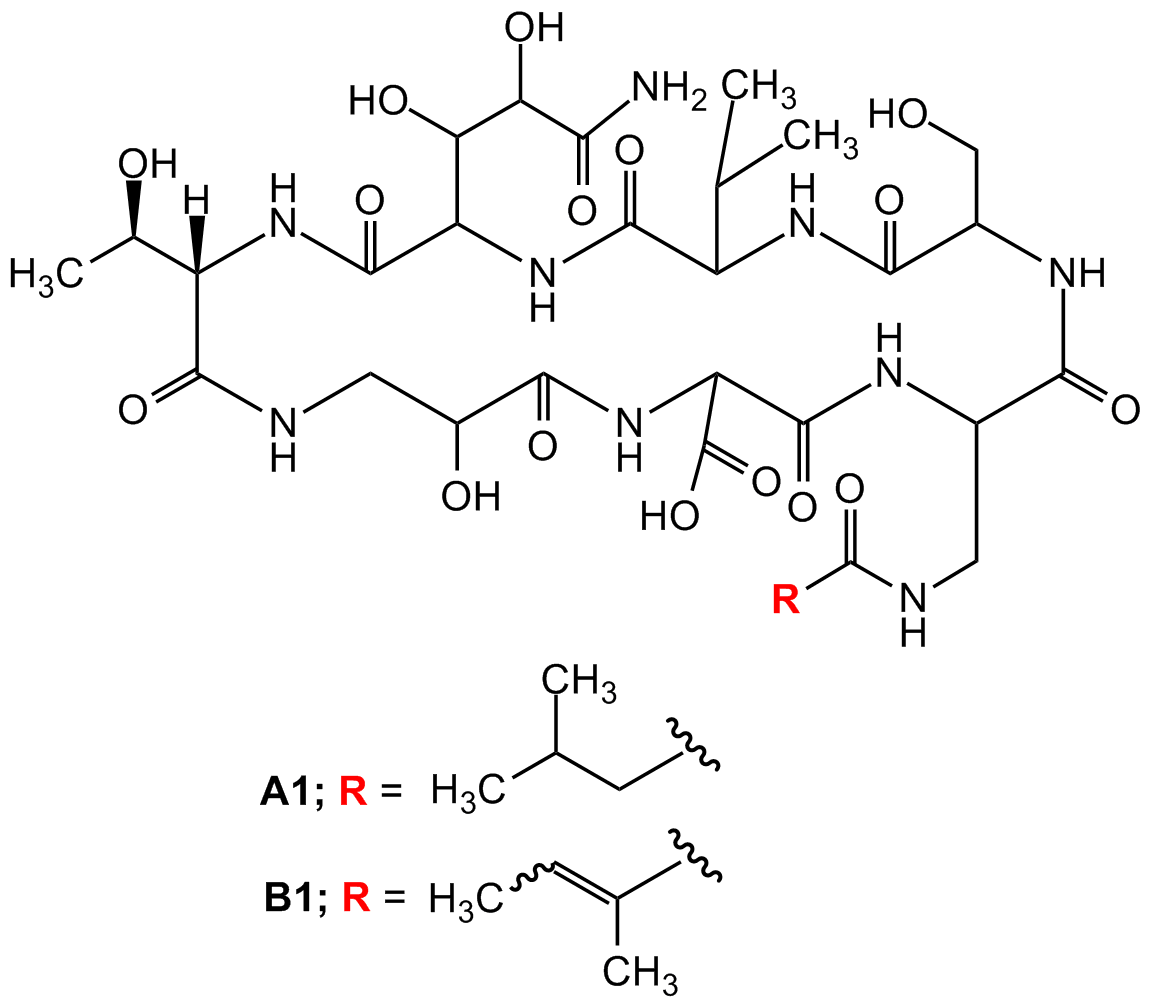
Antibiotic GE23077A1/Antibiotic GE23077B1 Mixture
Appearance:
White to off-white powder.
CAS:
752989-46-1 [Mixture A1/B1]752989-45-0 [A1]752989-44-9 [B1]
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light.Protect from light when in solution.
InChi:
InChI=1S/C31H51N9O16.C31H49N9O16/c2*1-6-11(4)23(47)33-7-13-24(48)36-14(9-41)25(49)37-16(10(2)3)28(52)39-18(20(44)21(45)22(32)46)29(53)38-17(12(5)42)27(51)34-8-15(43)26(50)40-19(31(55)56)30(54)35-13/h10-21,41-45H,6-9H2,1-5H3,(H2,32,46)(H,33,47)(H,34,51)(H,35,54)(H,36,48)(H,37,49)(H,38,53)(H,39,52)(H,40,50)(H,55,56);6,10,12-21,41-45H,7-9H2,1-5H3,(H2,32,46)(H,33,47)(H,34,51)(H,35,54)(H,36,48)(H,37,49)(H,38,53)(H,39,52)(H,40,50)(H,55,56)/b;11-6+
InChiKey:
LHEVPJVFNYHCLJ-USIKRYRBSA-N
Long Description:
Chemical. CAS: 752989-46-1 [Mixture A1/B1], 752989-45-0 [A1]752989-44-9 [B1]. Formula: C31H51N9O16 [A1], C31H49N9O16 [B1]. MW: 805.8 [A1]803.8 [B1]. Isolated from Actinomadura sp. Cyclic heptapeptide antibiotic. Potent and selective inhibitor of bacterial RNA polymerase (RNAP). Inhibits Gram-positive (Bacillus subtilis) and Gram-negative (Escherichia coli) RNAPs with IC50 ~20nM, whereas it is not active on E. coli DNA polymerase or on eukaryotic (wheat germ) RNAP II (IC50 values >100µM). Even though of its potent activity in vitro on purified bacterial RNAPs, it shows a narrow spectrum of antimicrobial activity in vivo on Gram-positive and Gram-negative bacteria, due to lack of memmbrane permeability. Acts at the level of transcription initiation. Binds directly to the bacterial RNA polymerase (RNAP) active-center 'i' and 'i+1' nucleotide binding sites, preventing the binding of initiating nucleotides and thereby preventing transcription initiation. Tool for mechanistic studies on bacterial RNAP and for potential anti-infective drug discovery.
Molecular Formula:
C31H51N9O16 [A1]
C31H49N9O16 [B1]
Molecular Weight:
805.8 [A1]803.8 [B1]
Other data:
Purity Note:The purity is referred exclusively to the main congener(s), the sample also contains minor related congeners. See: Antibiotics GE23077, novel inhibitors of bacterial RNA polymerase. I. Taxonomy, isolation and characterization: I. Ciciliato, et al.; J. Antibiot. 57, 210 (2004)
Package Type:
Vial
Product Description:
Cyclic heptapeptide antibiotic. Potent and selective inhibitor of bacterial RNA polymerase (RNAP). Inhibits Gram-positive (Bacillus subtilis) and Gram-negative (Escherichia coli) RNAPs with IC50 ~20nM, whereas it is not active on E. coli DNA polymerase or on eukaryotic (wheat germ) RNAP II (IC50 values >100µM). Even though of its potent activity in vitro on purified bacterial RNAPs, it shows a narrow spectrum of antimicrobial activity in vivo on Gram-positive and Gram-negative bacteria, due to lack of membrane permeability. Acts at the level of transcription initiation. Binds directly to the bacterial RNA polymerase (RNAP) active-center 'i' and 'i+1' nucleotide binding sites, preventing the binding of initiating nucleotides and thereby preventing transcription initiation. Tool for mechanistic studies on bacterial RNAP and for potential anti-infective drug discovery.
Purity:
>70% (HPLC, for A1/B1)
SMILES:
O=C1NC(CNC(/C(C)=C/C)=O)C(NC(CO)C(NC(C(C)C)C(NC(C(NC(C(C)O)C(NCC(O)C(NC1C(O)=O)=O)=O)=O)C(C(C(N)=O)O)O)=O)=O)=O.O=C2NC(CNC(C(C)CC)=O)C(NC(CO)C(NC(C(C)C)C(NC(C(NC(C(C)O)C(NCC(O)C(NC2C(O)=O)=O)=O)=O)C(C(C(N)=O)O)O)=O)=O)=O
Solubility Chemicals:
Soluble in DMSO or water.
Source / Host:
Isolated from Actinomadura sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
Antibiotics GE23077, novel inhibitors of bacterial RNA polymerase. I. Taxonomy, isolation and characterization: I. Ciciliato, et al.; J. Antibiot. 57, 210 (2004) | Mode of action of the microbial metabolite GE23077, a novel potent and selective inhibitor of bacterial RNA polymerase: E. Sarubbi, et al.; Eur. J. Biochem. 271, 3146 (2004) | Antibiotics GE23077, novel inhibitors of bacterial RNA polymerase. II. Structure elucidation: A. Marazzi, et al.; J. Antibiot. 58, 260 (2005) | Antibiotics GE23077, novel inhibitors of bacterial RNA polymerase. Part 3: Chemical derivatization: R. Mariani, et al.; Bioorg. Med. Chem. Lett. 15, 3748 (2005) | GE23077 binds to the RNA polymerase 'i' and 'i+1' sites and prevents the binding of initiating nucleotides: Y. Zhang, et al.; Elife 3, e02450 (2014)
Related Products
| Product Name | Product Code | Supplier | Actagardin | AG-CN2-0300 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GE2270A | AG-CN2-0303 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GE2270 D2 | AG-CN2-0304 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NAI-107 [Microbisporicin] | AG-CN2-0307 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NAI-112 | AG-CN2-0309 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||