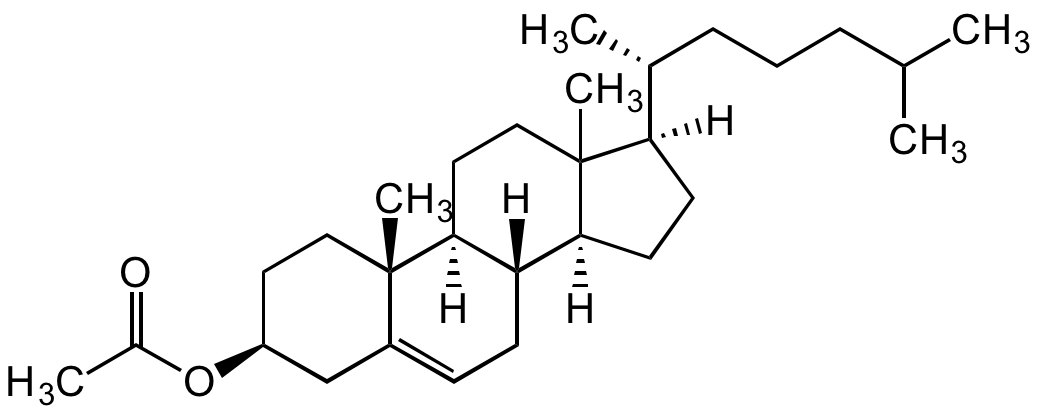
Cholesteryl acetate
Product Code:
CDX-C0359
CDX-C0359
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
+4 °C
+4 °C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-C0359-G050 | 50 g | £150.00 |
Quantity:
| CDX-C0359-G100 | 100 g | £231.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
Cholesterol acetate; 3beta-Acetoxy-5-cholestene; 3beta-Hydroxy-5-cholestene 3-acetate; 5-Cholesten-3beta-ol 3-acetate; NSC 8799
Appearance:
White solid.
CAS:
604-35-3
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C29H48O2/c1-19(2)8-7-9-20(3)25-12-13-26-24-11-10-22-18-23(31-21(4)30)14-16-28(22,5)27(24)15-17-29(25,26)6/h10,19-20,23-27H,7-9,11-18H2,1-6H3/t20-,23+,24+,25-,26+,27+,28+,29?/m1/s1
InChiKey:
XUGISPSHIFXEHZ-JWAONGLTSA-N
Long Description:
Chemical. CAS: 604-35-3. Formula: C29H48O2. MW: 428.69. Synthetic. Cholesteryl acetate is a normal human cholesteryl ester present in diverse fluids and organs. Cholesteryl acetate is also present in foods. Food oxidation affects the quality and safety of the human diet by generating compounds with biological activities that can adversely affect health. In particular the susceptibility of cholesterol to oxidation is well known, certain products of cholesterol oxidation have been reported to produce cytotoxic, angiotoxic and carcinogenic effects. Cholesteryl ester (CE) is the major transport and storage form of cholesterol in lipoprotein particles and most cell types. Molecular composition of CE species is of high interest for arteriosclerosis research, i. e. as components of lipoprotein subclasses or in studies investigating the mechanisms involved in the generation of lipid laden foam cells. Thus, it has been shown that CE species in circulating plasma are strongly correlated with development of coronary heart disease. Cholesteryl acetate is used in cosmetics or pharmaceuticals.
MDL:
MFCD00003636
Molecular Formula:
C29H48O2
Molecular Weight:
428.69
Package Type:
Vial
Product Description:
Cholesteryl acetate is a normal human cholesteryl ester present in diverse fluids and organs. Cholesteryl acetate is also present in foods. Food oxidation affects the quality and safety of the human diet by generating compounds with biological activities that can adversely affect health. In particular the susceptibility of cholesterol to oxidation is well known, certain products of cholesterol oxidation have been reported to produce cytotoxic, angiotoxic and carcinogenic effects. Cholesteryl ester (CE) is the major transport and storage form of cholesterol in lipoprotein particles and most cell types. Molecular composition of CE species is of high interest for arteriosclerosis research, i. e. as components of lipoprotein subclasses or in studies investigating the mechanisms involved in the generation of lipid laden foam cells. Thus, it has been shown that CE species in circulating plasma are strongly correlated with development of coronary heart disease. Cholesteryl acetate is used in cosmetics or pharmaceuticals.
Purity:
>98% (HPLC)
SMILES:
C[C@H](CCCC(C)C)[C@@]1([H])CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](OC(C)=O)CC[C@]4(C)[C@@]3([H])CCC21C
Solubility Chemicals:
Soluble in chloroform.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
Documents
References
(1) P. Sawzik & B.M. Craven; Acta Cryst. 35, 895 (1979) | (2) E.G. Perkins, et al.; Lipids 16, 609 (1981) | (3) P.J. Dolphin, et al.; Atheroscler. 51, 109 (1984) | (4) B. Sjostrom, et al.; J. Pharm. Sci. 82, 584 (1993) | (5) B. Sjostrom, et al.; Int. J. Pharm. 94, 89 (1993) | (6) B. Sjostrom, et al.; Pharm. Res. 12, 39 (1995) | (7) F.P. Ballistreri, et al.; Steroids 71, 565 (2006) | (8) D.A. Aleksandrov, et al.; FEBS J. 273, 548 (2006) | (9) I. Gvozdovskyy & L. Lisetski; Europ. Phys. J. E 24, 211 (2007)
Related Products
| Product Name | Product Code | Supplier | 7-Dehydrocholesterol | CDX-D0331 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7beta-Hydroxy-cholesteryl-bishemisuccinate-diethanolamine salt | CDX-H0123 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||