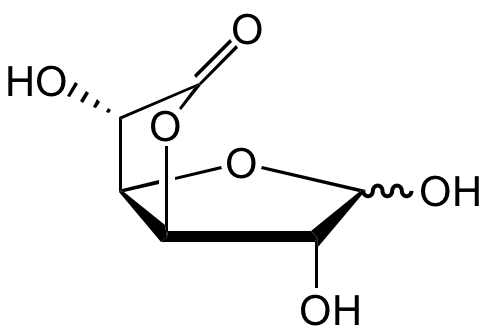
Glucuronolactone
Product Code:
CDX-G0044
CDX-G0044
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
Short Term: +20°C. Long Term: +20°C
Short Term: +20°C. Long Term: +20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-G0044-G025 | 25 g | £65.00 |
Quantity:
| CDX-G0044-G250 | 250 g | £157.00 |
Quantity:
| CDX-G0044-G500 | 500 g | £280.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
D-(+)-Glucuronic acid gamma-lactone; D-(+)-Glucurono-6,3-lactone; D-Glucurono-6,3-lactone; D-Glucurone
Appearance:
White powder.
CAS:
32449-92-6
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C6H8O6/c7-1-3-4(12-5(1)9)2(8)6(10)11-3/h1-5,7-9H/t1-,2+,3-,4-,5?/m1/s1
InChiKey:
OGLCQHRZUSEXNB-VGASXLIASA-N
Long Description:
Chemical. CAS: 32449-92-6. Formula: C6H8O6. MW: 176.12. Synthetic. Naturally occurring carbohydrate derivative that is an important structural component of nearly all connective tissues and is also found in many plant gums. Metabolized to glucaric acid, xylitol, and L-xylulose, and humans may also be able to use glucuronolactone as a precursor for ascorbic acid synthesis. Used as a detoxicant. The liver uses glucose to create glucuronolactone, which inhibits the enzyme B-glucuronidase (metabolizes glucuronides), which should cause blood-glucuronide levels to rise. Glucuronides combine with toxic substances, such as morphine and depot medroxyprogesterone acetate, by converting them to water-soluble glucuronide-conjugates which are excreted in the urine. Used as building block and starting reagent for synthesis of drugs, optically active glucopyranoses and long-chain alkyl glucofuranosides.
MDL:
MFCD00135622
Molecular Formula:
C6H8O6
Molecular Weight:
176.12
Package Type:
Vial
Product Description:
Naturally occurring carbohydrate derivative that is an important structural component of nearly all connective tissues and is also found in many plant gums. Metabolized to glucaric acid, xylitol, and L-xylulose, and humans may also be able to use glucuronolactone as a precursor for ascorbic acid synthesis. Used as a detoxicant. The liver uses glucose to create glucuronolactone, which inhibits the enzyme B-glucuronidase (metabolizes glucuronides), which should cause blood-glucuronide levels to rise. Glucuronides combine with toxic substances, such as morphine and depot medroxyprogesterone acetate, by converting them to water-soluble glucuronide-conjugates which are excreted in the urine. Used as building block and starting reagent for synthesis of drugs, optically active glucopyranoses and long-chain alkyl glucofuranosides.
Purity:
>98% (HPLC)
SMILES:
O[C@@H]1[C@@H](OC2=O)[C@@H]([C@@H]2O)OC1O
Solubility Chemicals:
Soluble in water.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +20°C.
Documents
References
(1) C.A. Marsh; Biochem. J. 99, 22 (1966) | (2) S.H. Kim, et al.; Acta Crystallogr. 22, 733 (1967) | (3) L. Trahan, et al.; Rev. Can. Biol. 29, 7 (1970) | (4) T. Kuzuya, et al.; Endocrinol. Jpn. 20, 369 (1973) | (5) A. Kirschning, et al.; Bioorg. Med. Chem. Lett. 7, 903 (1997) | (6) S. Suzuki, et al.; J. Chromatogr. Sci. 36, 357 (1998) | (7) A.F. Glawar, et al.; Chemistry 18, 9341 (2012)
Related Products
| Product Name | Product Code | Supplier | 2,2-Dimethylcyclopentanone | CDX-D0333 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difluoromethyl phenyl sulfone | CDX-D0401 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3,4-Epoxycyclohex-1-en | CDX-E0058 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1,1'-(1,2-Ethanediyl)bis-1H-1,2,4-triazole | CDX-E0066 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4-Fluororesorcinol | CDX-F0047 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5-Hydroxynicotinic acid | CDX-H0052 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||