Storage:
Short Term: +20°C. Long Term: +4°C
No additional charges, what you see is what you pay! *
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available!
Contact us to find what you can save.
This product comes from:
Switzerland.
Typical lead time:
7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
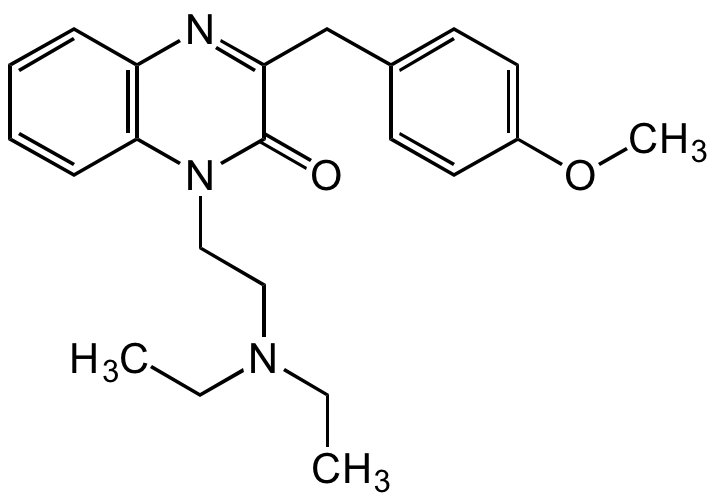
1-[2-(Diethylamino)ethyl]-3-(p-methoxybenzyl)-2-quinoxalone; Spadon; Spasmium
White or slightly yellow, fine powder.
23465-76-1
32160000
liquid
GHS07
Keep cool and dry.Protect from light and moisture.
H302
InChI=1S/C22H27N3O2/c1-4-24(5-2)14-15-25-21-9-7-6-8-19(21)23-20(22(25)26)16-17-10-12-18(27-3)13-11-17/h6-13H,4-5,14-16H2,1-3H3
MSPRUJDUTKRMLM-UHFFFAOYSA-N
Chemical. CAS: 23465-76-1. Formula: C22H27N3O2. MW: 365.47. Synthetic. Quinoxaline compound, originally developed as a spasmolytic drug. Acts as an N-type calcium channel blocker, competitive AMPA receptor antagonist, and non-competitive NMDA receptor antagonist. Antioxidant agent through partial prevention of the formation and the highly active scavenging of hydroxyl radicals. Used to treat inner ear diseases such as tinnitus. Shown to inhibit tumor-promoting factors and potentially useful as a chemotherapeutic agent.
MFCD00865257
C22H27N3O2
365.47
Vial
P264, P270, P301, P312, P330, P501
Quinoxaline compound, originally developed as a spasmolytic drug. Acts as an N-type calcium channel blocker, competitive AMPA receptor antagonist, and non-competitive NMDA receptor antagonist. Antioxidant agent through partial prevention of the formation and the highly active scavenging of hydroxyl radicals. Used to treat inner ear diseases such as tinnitus. Shown to inhibit tumor-promoting factors and potentially useful as a chemotherapeutic agent.
>99% (HPLC)
Warning
O=C1N(CCN(CC)CC)C2=C(C=CC=C2)N=C1CC3=CC=C(OC)C=C3
Soluble in DMSO (10 mg/ml). Insolube in water.
Synthetic.
Non-hazardous
Biochemical Reagents
12352200
Stable for at least 2 years after receipt when stored at +4°C.