MAPTAM
Product Code:
CDX-B0292
CDX-B0292
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
Short Term: +4°C. Long Term: -20°C
Short Term: +4°C. Long Term: -20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-B0292-M010 | 10 mg | £108.00 |
Quantity:
| CDX-B0292-M025 | 25 mg | £230.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
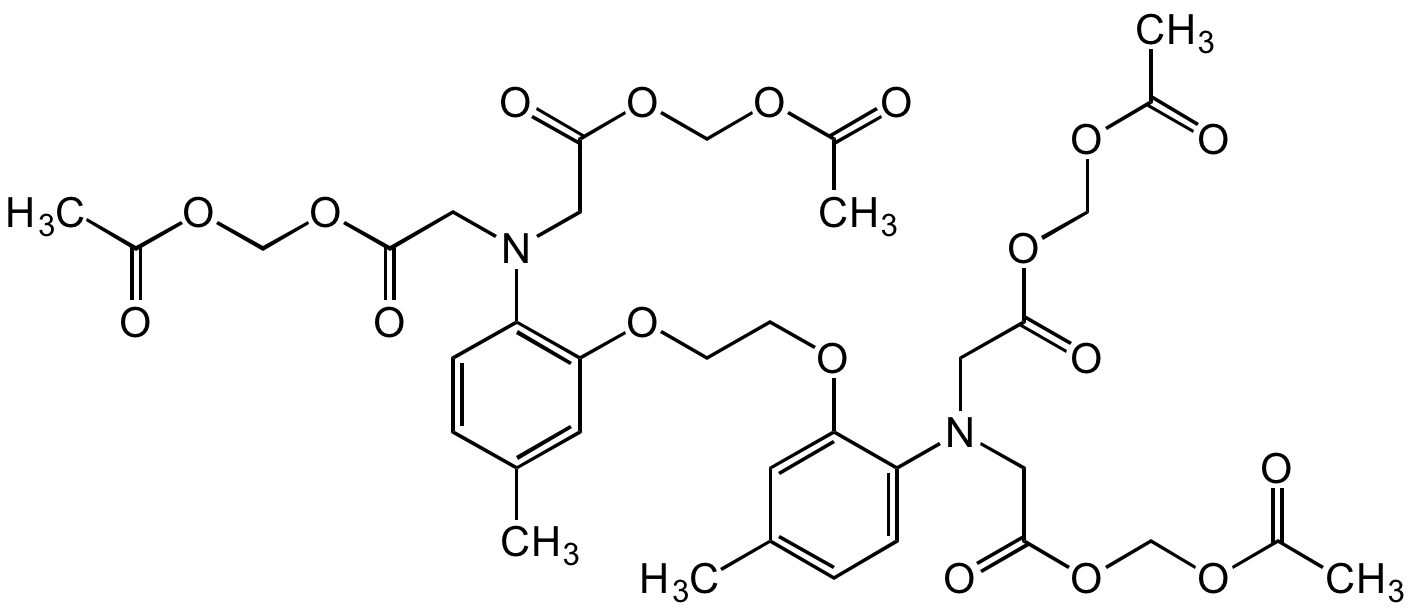
5,5'-Dimethyl-BAPTA-AM; 1,2-Bis(2-amino-5-methylphenoxy) ethane; 1,2-Bis(2-amino-5-methylphenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis(acetoxymethyl) ester
Appearance:
White to off-white powder.
CAS:
147504-94-7
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H315, H319, H335
InChi:
InChI=1S/C36H44N2O18/c1-23-7-9-29(37(15-33(43)53-19-49-25(3)39)16-34(44)54-20-50-26(4)40)31(13-23)47-11-12-48-32-14-24(2)8-10-30(32)38(17-35(45)55-21-51-27(5)41)18-36(46)56-22-52-28(6)42/h7-10,13-14H,11-12,15-22H2,1-6H3
InChiKey:
HEOJVQZWOLQUDR-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 147504-94-7. Formula: C36H44N2O18. MW: 792.74. Synthetic. MAPTAM is a cell permeable intracellular Ca2+ chelator that can be loaded non-invasive into cells by incubation. MAPTAM itself does not bind calcium, but once inside the cell is converted into it's derivative Dimethyl-BAPTA by cytoplasmic esterases. This type of calcium chelators are commonly used to form calcium buffers with well-defined calcium concentrations. By injecting the chelators into cells or by incubating cells with the AM ester form of the chelators, one can control the cytosolic calcium concentration, an important means to study the roles of calcium. Key advantages of these calcium chelators include relative insensitivity toward intracellular pH change and fast release of calcium. Recent studies have shown that BAPTA derivative might have microtubule disruptive activity (unrelated to it's calcium chelating activity) and should be used with caution in studies of cytoskeleton-related cell functions.
MDL:
MFCD00036856
Molecular Formula:
C36H44N2O18
Molecular Weight:
792.74
Package Type:
Vial
Precautions:
P261, P305, P351, P338
Product Description:
MAPTAM is a cell permeable intracellular Ca2+ chelator that can be loaded non-invasive into cells by incubation. MAPTAM itself does not bind calcium, but once inside the cell is converted into it's derivative Dimethyl-BAPTA by cytoplasmic esterases. This type of calcium chelators are commonly used to form calcium buffers with well-defined calcium concentrations. By injecting the chelators into cells or by incubating cells with the AM ester form of the chelators, one can control the cytosolic calcium concentration, an important means to study the roles of calcium. Key advantages of these calcium chelators include relative insensitivity toward intracellular pH change and fast release of calcium. Recent studies have shown that BAPTA derivative might have microtubule disruptive activity (unrelated to it's calcium chelating activity) and should be used with caution in studies of cytoskeleton-related cell functions.
Purity:
>94% (HPLC)
Signal word:
Warning
SMILES:
CC1=CC(OCCOC2=CC(C)=CC=C2N(CC(OCOC(C)=O)=O)CC(OCOC(C)=O)=O)=C(N(CC(OCOC(C)=O)=O)CC(OCOC(C)=O)=O)C=C1
Solubility Chemicals:
Soluble in DMSO, DMF, acetonitrile, ethyl acetate or chloroform.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) Biochemistry 19, 2396 (1980) | (2) R.Y. Tsein; Nature 290, 527 (1981) | (3) J.B. Lefkowith, et al.; J. Immunol. 149, 1729 (1992) | (4) K.A. Birch, et al.; J. Cell Biol. 118, 1501 (1992) | (5) E. Poch, et al.; Biochem. J. 290, 617 (1993) | (6) J.M. Dubinsky; Neurosci. Lett. 150, 129 (1993) | (7) T. Tiffert & V.L. Lew; J. Physiol. 505, 403 (1997) | (8) J.R. Wu-Wong, et al.; Clin. Sci. 103 Suppl.48, 418S (2002) | (9) A. Furuta, et al.; Endocr. J. 56, 235 (2009) | (10) R. Chen, et al.; Microb. Pathog. 83-84, 29 (2015)
Related Products
| Product Name | Product Code | Supplier | Arsenazo III | CDX-A0308 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluo-3 | CDX-F0056 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indo-1 pentapotassium salt | CDX-I0018 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||