No additional charges, what you see is what you pay! *
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available!
Contact us to find what you can save.
This product comes from:
Switzerland.
Typical lead time:
7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
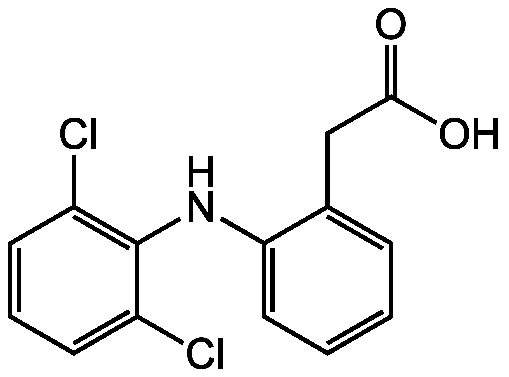
Voltaren; Diclofenacum; 2-[(2,6-Dichlorophenyl)amino]-benzeneacetic acid
White powder.
15307-86-5
6.1
32160000
liquid
GHS06
Keep cool and dry.Protect from light and moisture.
H301
InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19)
DCOPUUMXTXDBNB-UHFFFAOYSA-N
Chemical. CAS: 15307-86-5. Formula: C14H11Cl2NO2. MW: 296.15. Synthetic Nonsteroidal anti-inflammatory drug (NSAID) taken or applied to reduce inflammation and as an analgesic reducing pain. The primary mechanism responsible for its anti-inflammatory, antipyretic, and analgesic action is thought to be inhibition of prostaglandin synthesis by inhibition of cyclooxygenase (COX). It also appears to exhibit bacteriostatic activity by inhibiting bacterial DNA synthesis. Can be used as a reference compound.
MFCD00056694
C14H11Cl2NO2
296.15
Vial
III
P270, P264, P301, P310, P330, P405, P501
Nonsteroidal anti-inflammatory drug (NSAID) taken or applied to reduce inflammation and as an analgesic reducing pain. The primary mechanism responsible for its anti-inflammatory, antipyretic, and analgesic action is thought to be inhibition of prostaglandin synthesis by inhibition of cyclooxygenase (COX). It also appears to exhibit bacteriostatic activity by inhibiting bacterial DNA synthesis. Can be used as a reference compound.
>98% (HPLC)
Danger
OC(=O)CC1=CC=CC=C1NC1=C(Cl)C=CC=C1Cl
Sligthly soluble in water (2.37 mg/L).
Synthetic
Excepted Quantity
UN 2811
Biochemical Reagents
12352200
Stable for at least 2 years after receipt when stored at +20°C.