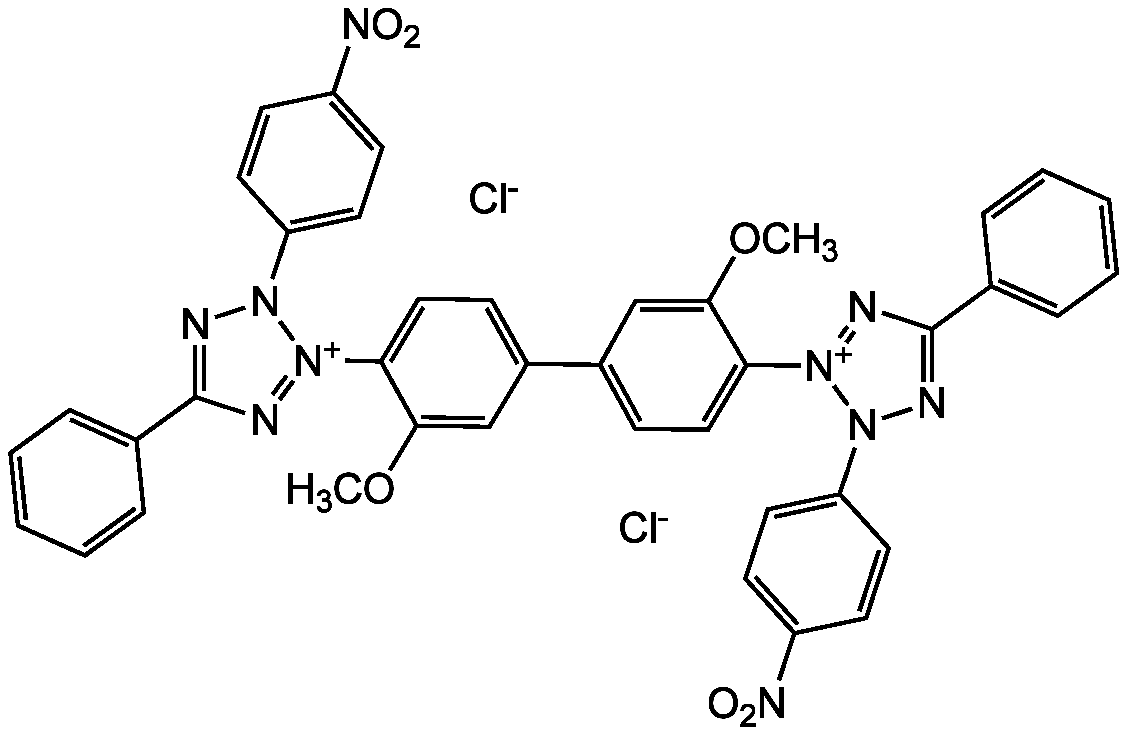
Nitrotetrazolium blue chloride
Product Code:
CDX-N0009
CDX-N0009
Regulatory Status:
RUO
RUO
Shipping:
AMBIENT
AMBIENT
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-N0009-M100 | 100 mg | £34.00 |
Quantity:
| CDX-N0009-G001 | 1 g | £121.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
NBT; p-Nitro-Blue tetrazolium chloride; p-Nitrotetrazolium blue; Nitro BT
Appearance:
Yellow powder.
CAS:
298-83-9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H302, H315, H319, H335
InChi:
InChI=1S/C40H30N10O6.2ClH/c1-55-37-25-29(13-23-35(37)47-43-39(27-9-5-3-6-10-27)41-45(47)31-15-19-33(20-16-31)49(51)52)30-14-24-36(38(26-30)56-2)48-44-40(28-11-7-4-8-12-28)42-46(48)32-17-21-34(22-18-32)50(53)54;;/h3-26H,1-2H3;2*1H/q+2;;/p-2
InChiKey:
FSVCQIDHPKZJSO-UHFFFAOYSA-L
Long Description:
Chemical. CAS: 298-83-9. Formula: C40H30Cl2N10O6. MW: 817.2. Synthetic. NADPH-diaphorase substrate that competitively inhibits NOS (nitric oxide synthase). Well-known scavenger of superoxide anions. Dye that is used for detection of alkaline phosphatase in combination with 5-bromo-4-chloro-3-indoxyl phosphate (BCIP). This substrate system produces an insoluble NBT diformazan end product that is blue in color and can be observed visually. When used with BCIP, it is suitable for detection of alkaline phosphatase in western blots, for immunohistological staining procedures and for colorimetric indication of bacterial infection in blood samples. Used as a redox indicator for enzymatic reactions including dehydrogenases, threonine deaminase, glucose-6-phosphate dehydrogenase, phosphofructokinase on polyacrylamide gels, oxidases on polyacrylamide gels and pentose shunt dehydrogenses. The NBT/BCIP reaction is also used for colorimetric/spectrophotometric activity assays of oxidoreductases. One application is in activity stains in gel electrophoresis, such as with the mitochondrial electron transport chain complexes.
MDL:
MFCD00012159
Molecular Formula:
C40H30Cl2N10O6
Molecular Weight:
817.2
Package Type:
Vial
Precautions:
P261, P305, P351, P338
Product Description:
NADPH-diaphorase substrate that competitively inhibits NOS (nitric oxide synthase). Well-known scavenger of superoxide anions. Dye that is used for detection of alkaline phosphatase in combination with 5-bromo-4-chloro-3-indoxyl phosphate (BCIP). This substrate system produces an insoluble NBT diformazan end product that is blue in color and can be observed visually. When used with BCIP, it is suitable for detection of alkaline phosphatase in western blots, for immunohistological staining procedures and for colorimetric indication of bacterial infection in blood samples. Used as a redox indicator for enzymatic reactions including dehydrogenases, threonine deaminase, glucose-6-phosphate dehydrogenase (G6PDH), phosphofructokinase on polyacrylamide gels, oxidases on polyacrylamide gels and pentose shunt dehydrogenses. The NBT/BCIP reaction is also used for colorimetric/spectrophotometric activity assays of oxidoreductases. One application is in activity stains in gel electrophoresis, such as with the mitochondrial electron transport chain complexes.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
[Cl-].[Cl-].COC1=CC(=CC=C1[N+]1=NC(=NN1C1=CC=C(C=C1)[N+]([O-])=O)C1=CC=CC=C1)C1=CC=C(C(OC)=C1)[N+]1=NC(=NN1C1=CC=C(C=C1)[N+]([O-])=O)C1=CC=CC=C1
Solubility Chemicals:
Soluble in water.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) D.G. Nathan, et al.; J. Clin. Invest. 48, 1895 (1969) | (2) J. McGadey; Histochem. Histochem. Histochim. 23, 180 (1970) | (3) R. Freeman & B. King; J. Clin. Pathol. 25, 912 (1972) | (4) D.A. Knecht & R.L. Dimond; Anal. Biochem. 136, 180 (1984) | (5) Y. Nisimoto, et al.; J. Biol. Chem. 261, 285 (1986) | (6) K. Nichols, et al.; Neuroscience 51, 791 (1992) | (7) C. Libon, et al.; J. Leukoc. Biol. 53, 93 (1993) | (8) C.K. Mittal; Biochem. Biophys. Res. Commun. 193, 126 (1993) | (9) M. De la Fuente, et al.; Comp. Immunol. Microbiol. Infect. Dis. 16, 29 (1993) | (10) A. Pistelli, et al.; Biochem. Pharmacol. 47, 1737 (1994) | (11) A. Trinhle, et al.; BioTechniques 42|756 (2007) | (12) J.M. Ross; J. Visual. Exp. 57, e3266 (2011) | (13) Z.A. Siddiqi, et al.; Eur. J. Med. Chem. 57, 102 (2012)
Related Products
| Product Name | Product Code | Supplier | Dihydroethidium | CDX-D0094 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Naphthol AS-E phosphate | CDX-N0125 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||