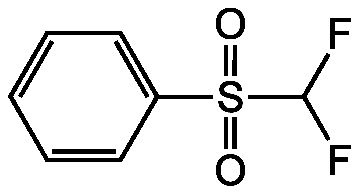
Difluoromethyl phenyl sulfone
Product Code:
CDX-D0401
CDX-D0401
Regulatory Status:
RUO
RUO
Shipping:
AMBIENT
AMBIENT
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-D0401-GG25 | 2.5 g | £102.00 |
Quantity:
| CDX-D0401-G010 | 10 g | £280.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
Phenyl difluoromethyl sulfone
Appearance:
Colorless to light yellow semi-solid or solid.
CAS:
1535-65-5
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H315, H319, H335
InChi:
InChI=1S/C7H6F2O2S/c8-7(9)12(10,11)6-4-2-1-3-5-6/h1-5,7H
InChiKey:
LRHDNAVPELLXDL-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 1535-65-5. Formula: C7H6F2O2S. MW: 192.2. Synthetic. Building block for synthesis. Has been used for nucleophilic difluoro(phenylsulfonyl)methylation of carbonyls, reductive silylation and the preparation of trifluoro- and difluoromethylsilanes, fluoroalkylation/chloroalkylation of alpha,beta-enones, arynes, acetylenic ketones and other Michael acceptors and difluoromethylation of primary alkyl halides . It is also used in preparation of alpha-difluoromethyl amines, anti-difluoropropanediols, beta-difluoromethylated and beta-difluoromethylenated alcohols and amines, difluoroalkenes, difluoromethyl alcohol derivatives and fluoromethylated vicinal ethylenediamines.
MDL:
MFCD01050170
Molecular Formula:
C7H6F2O2S
Molecular Weight:
192.2
Package Type:
Vial
Precautions:
P261, P305, P351, P338
Product Description:
Building block for synthesis. Has been used for nucleophilic difluoro(phenylsulfonyl)methylation of carbonyls, reductive silylation and the preparation of trifluoro- and difluoromethylsilanes, fluoroalkylation/chloroalkylation of alpha,beta-enones, arynes, acetylenic ketones and other Michael acceptors and difluoromethylation of primary alkyl halides . It is also used in preparation of alpha-difluoromethyl amines, anti-difluoropropanediols, beta-difluoromethylated and beta-difluoromethylenated alcohols and amines, difluoroalkenes, difluoromethyl alcohol derivatives and fluoromethylated vicinal ethylenediamines.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
FC(F)S(=O)(=O)C1=CC=CC=C1
Solubility Chemicals:
Soluble in chloroform.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) G.K.S. Prakash, et al.; J. Org. Chem. 68, 4457 (2003) | (2) G.K.S. Prakash, et al.; Angew. Chem. 42, 5216 (2003) | (3) G.K.S. Prakash, et al.; Angew. Chem. 43, 5203 (2004) | (4) G.K.S. Prakash, et al.; Org. Lett. 6, 4315 (2004) | (5) Y. Li & J. Hu; Angew. Chem. 44, 5882 (2005) | (6) G.K.S. Prakash, et al.; Europ. J. Org. Chem. 11, 2218 (2005) | (7) C. Ni, et al.; Angew. Chem. 46, 786 (2007) | (8) J. Liu, et al.; J. Org. Chem. 72, 3119 (2007) | (9) C. Ni, et al.; J. Org. Chem. 73, 5699 (2008) | (10) M. Hu, et al.; J. Fluor. Chem. 155, 52 (2013)
Related Products
| Product Name | Product Code | Supplier | 2,2-Dimethylcyclopentanone | CDX-D0333 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (S,S)-(-)-1,4-Dimethoxy-2,3-butanediol | CDX-D0434 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3,4-Epoxycyclohex-1-en | CDX-E0058 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1,1'-(1,2-Ethanediyl)bis-1H-1,2,4-triazole | CDX-E0066 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4-Fluororesorcinol | CDX-F0047 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5-Hydroxynicotinic acid | CDX-H0052 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||