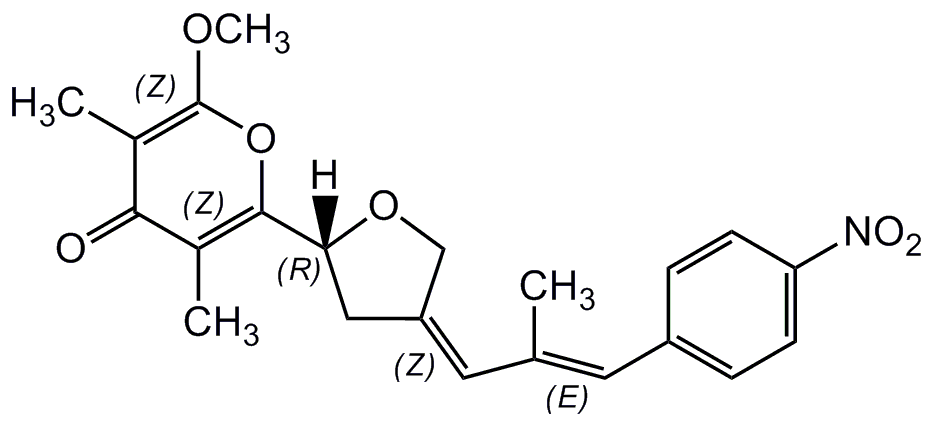
Aureothin
Product Code:
BVT-0303
BVT-0303
Regulatory Status:
RUO
RUO
Shipping:
20°C
20°C
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| BVT-0303-M001 | 1 mg | £245.00 |
Quantity:
| BVT-0303-M005 | 5 mg | £750.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
Mycolutein; Distacin; JA 2814K; Antibiotic 74A; BRN 0058476
Appearance:
Yellow solid.
CAS:
2825-00-5
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Protect from light when in solution.
Hazards:
H300, H310, H319, H332
InChi:
InChI=1S/C22H23NO6/c1-13(9-16-5-7-18(8-6-16)23(25)26)10-17-11-19(28-12-17)21-14(2)20(24)15(3)22(27-4)29-21/h5-10,19H,11-12H2,1-4H3/b13-9+,17-10-
InChiKey:
GQKXCBCSVYJUMI-RCGCBBHSSA-N
Long Description:
Chemical. CAS: 2825-00-5. Formula: C22H23NO6. MW: 397.4. Isolated from Streptomyces thioluteus. Oxidoreductase inhibitor. Antitrypanosomal, antibacterial, antifungal, insecticidal and pesticidal. Antitumor compound.
MDL:
MFCD01694034
Molecular Formula:
C22H23NO6
Molecular Weight:
397.4
Package Type:
Plastic Vial
PG:
III
Precautions:
P261, P262, P280, P301, P310, P302, P350, P312
Product Description:
NADH dehydrogenase (NADH-Coenzyme Q oxidoreductase; Complex I) inhibitor. Inhibits mitochondrial respiration and oxidative phosphorylation (OXPHOS). Antitrypanosomal, antibacterial, antifungal, insecticidal and pesticidal. Antitumor compound. Potent anti-HIV agent.
Purity:
>96% (HPLC)
Signal word:
Danger
SMILES:
COC1=C(C)C(=O)C(C)=C(O1)C1CC(CO1)=CC(C)=CC1=CC=C(C=C1)[N+]([O-])=O
Solubility Chemicals:
Soluble in 100% ethanol, methanol, DMSO, dimethylformamide, dicholormethane or acetone.
Source / Host:
Isolated from Streptomyces thioluteus.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
Documents
References
The structure of aureothin, a nitro compound obtained from Streptomyces thioluteus: Y. Hirata, et al.; Tetrahedron 14, 252 (1961) | Identification of mycolutein and pulvomycin as aureothin and labilomycin respectively: J.L. Schwartz, et al.; J. Antibiot. 29, 236 (1976) | Two binding sites of inhibitors in NADH: ubiquinone oxidoreductase (complex I). Relationship of one site with the ubiquinone-binding site of bacterial glucose:ubiquinone oxidoreductase: T. Friedrich, et al.; Eur. J. Biochem. 219, 691 (1994) | Absolute configuration of (+)-aureothin: A toxic Metabolite posessing gamma-pyrone unit: Y. Ishibashi, et al.; Bull. Chem. Soc. Jpn. 68, 3643 (1995) | Gamma-pyrone compounds with selective and potent anti-Helicobacter pylori activity: M. Taniguchi, et al.; J. Antibiot. 53, 844 (2000) | Dissection of the late steps in aureothin biosynthesis: M. M?ller, et al.; ChemBioChem 7, 37 (2006) | New aureothin derivative, alloaureothin, from Streptomyces sp. MM23: J.Y. Ueda, et al.; J. Antibiot. 60, 321 (2007) | Chemoenzymatic total synthesis of the antiproliferative polyketide (+)-(R)-aureothin: M. Werneburg & C. Hertweck; Chembiochem 9, 2064 (2008) | Selective and potent in vitro antitrypanosomal activities of ten microbial metabolites: K. Otoguro, et al.; J. Antibiot. 61, 372 (2008) | Natural lipophilic inhibitors of mitochondrial complex I are candidate toxins for sporadic neurodegenerative tau pathologies: M. H?llerhagen, et al.; Exp. Neurol. 220, 133 (2009) | Evolution of metabolic diversity in polyketide-derived pyrones: using the non-colinear Aureothin assembly line as a model system: B. Busch, et al.; Phytochemistry 70, 1833 (2009) | Exploiting enzymatic promiscuity to engineer a focused library of highly selective antifungal and antiproliferative Aureothin analogues: M. Werneburg, et al.; J. Am. Chem. Soc. 132, 10407 (2010) | Potent inhibition of HIV replication in primary human cells by novel synthetic polyketides inspired by Aureothin: A. Herrmann, et al.; Sci. Rep. 10, 1326 (2020)
Related Products
| Product Name | Product Code | Supplier | Pimprinine | BVT-0297 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|