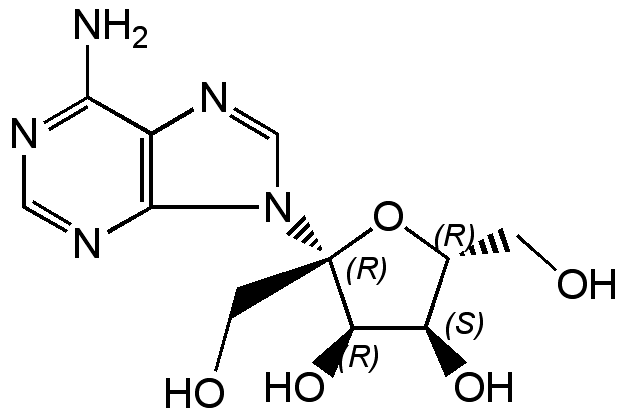
Psicofuranine
Product Code:
BVT-0284
BVT-0284
Regulatory Status:
RUO
RUO
Shipping:
20°C
20°C
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| BVT-0284-M001 | 1 mg | £95.00 |
Quantity:
| BVT-0284-M005 | 5 mg | £350.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
Angustmycin C; U-9586; 9-beta-D-Psicofuranosyl-9H-purin-6-amine
Appearance:
White to off-white solid.
CAS:
1874-54-0
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Avoid freeze/thaw cycles.
Hazards:
H302, H312, H319
InChi:
InChI=1S/C11H15N5O5/c12-9-6-10(14-3-13-9)16(4-15-6)11(2-18)8(20)7(19)5(1-17)21-11/h3-5,7-8,17-20H,1-2H2,(H2,12,13,14)/t5-,7-,8-,11-/m1/s1
InChiKey:
BNZYRKVSCLSXSJ-IOSLPCCCSA-N
Long Description:
Chemical. CAS: 1874-54-0. Formula: C11H15N5O5. MW: 297.3. Isolated from Streptomyces sp. S 2113. Nucleoside antibiotic. Antitumor compound. Xanthosine monophosphate (XMP) aminase inhibitor. Antimetabolite of the purine biosynthesis.
MDL:
MFCD01674066
Molecular Formula:
C11H15N5O5
Molecular Weight:
297.3
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P302, P352, P312
Product Description:
Nucleoside antibiotic. Antitumor compound. Xanthosine monophosphate (XMP) aminase inhibitor. Antimetabolite of the purine biosynthesis.
Purity:
>98% (HPLC, TLC)
Signal Word:
Warning
SMILES:
NC1=NC=NC2=C1N=CN2[C@]1(CO)O[C@H](CO)[C@@H](O)[C@H]1O
Solubility Chemicals:
Soluble in DMSO; poorly soluble in methanol.
Source / Host:
Isolated from Streptomyces sp. S 2113.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
Documents
References
Studies on angustmycins VIII. The structure of angustmycin C: H. Y?ntsen; J. Antibiot. 11A, 244 (1958) | Psicofuranine I. Discovery, isolation, and properties: T.E. Eble, et al.; Antibiot. Chemother. 9, 419 (1959) | Psicofuranine. VI. Antitumor and toxicopathological studies: J.S. Evans & J.E. Gray; Antibiot. Chemother. 9, 675 (1959) | Mechanism of action of psicofuranine: L.J. Hanka; J. Bacteriol. 80, 30 (1960) | Inhibition of parental and mutant xanthosine 5'-phosphate aminases by psicofuranine: S. Udaka & H.S. Moyed; J. Biol. Chem. 238, 2797 (1963) | Formation of an adenylxanthosinemonophosphate intermediate by xanthosine 5'-phosphate aminase and its inhibition by psicofuranine: T.T. Fukuyama; J. Biol. Chem. 241, 4745 (1966) | Nucleoside antibiotics: structure, biological activity, and biosynthesis: K. Isono; J. Antibiot. 41, 1711 (1988), (Review) | Role of purine biosynthetic intermediates in response to folate stress in Escherichia coli: C.E. Rohlman & R.G. Matthews; J. Bacteriol. 172, 7200 (1990) | Plasmodium falciparum: isolation and characterisation of a gene encoding protozoan GMP synthase: G.A. McConkey; Exp. Parasitol. 94, 23 (2000)