DAPI dihydrochloride Solution (10 mM in water)
Product Code:
CDX-D1217
CDX-D1217
Regulatory Status:
RUO
RUO
Shipping:
AMBIENT
AMBIENT
Storage:
Short term storage:-20°C. Long term storage:-20°C.
Short term storage:-20°C. Long term storage:-20°C.
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-D1217-M005 | 5 mg | £115.00 |
Quantity:
| CDX-D1217-M010 | 10 mg | £167.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride; 4',6-Diamidino-2-phenylindole dihydrochloride; FxCycle Violet
Appearance:
Liquid.
CAS:
28718-90-3
Concentration:
10mM in water
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light and moisture.
Hazards:
H303, H313, H340
InChi:
InChI=1S/C16H15N5.2ClH/c17-15(18)10-3-1-9(2-4-10)13-7-11-5-6-12(16(19)20)8-14(11)21-13;;/h1-8,21H,(H3,17,18)(H3,19,20);2*1H
InChiKey:
FPNZBYLXNYPRLR-UHFFFAOYSA-N
Long Description:
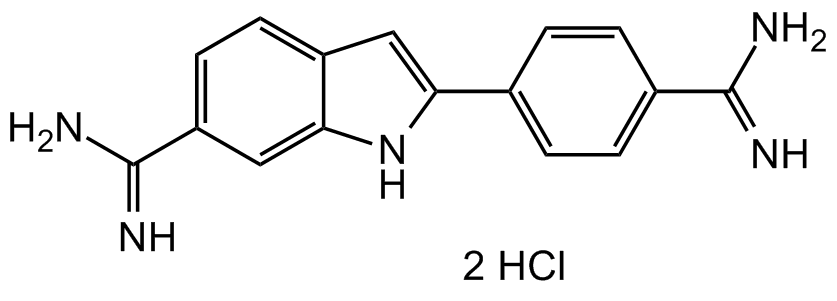
Chemical. CAS: 28718-90-3. Formula: C16H15N5 . 2HCl. MW: 350.25. DAPI (4,6-Diamidino-2-phenylindole dihydrochloride) is a cell permeable, fluorescent dye that is a minor groove-binding probe for DNA. Binds to the minor groove of double-stranded DNA (preferentially to AT rich DNA), forming a stable complex which fluoresces approximately 20 times greater than DAPI alone. DAPI is several times more sensitive than ethidium bromide for staining DNA in agarose gels. It may be used for photofootprinting of DNA, to detect annealed probes in blotting applications by specifically visualizing the double-stranded complex, and to study the changes in DNA and analyze DNA content during apoptosis using flow cytometry. DAPI staining has also been shown to be a sensitive and specific detection method for mycoplasma. Spectral Data: Excitation: lambdaex 340 nm; Emission: lambdaem 488 nm (only DAPI). Excitation: lambdaex 360nm; Emission: lambdaem 460nm (DAPI-DNA complex).
MDL:
MFCD00012681
Molecular Formula:
C16H15N5 . 2HCl
Molecular Weight:
350.25
Package Type:
Vial
Precautions:
P261, P271, P280
Product Description:
DAPI (4,6-Diamidino-2-phenylindole dihydrochloride) is a cell permeable, fluorescent dye that is a minor groove-binding probe for DNA. Binds to the minor groove of double-stranded DNA (preferentially to AT rich DNA), forming a stable complex which fluoresces approximately 20 times greater than DAPI alone. DAPI is several times more sensitive than ethidium bromide for staining DNA in agarose gels. It may be used for photofootprinting of DNA, to detect annealed probes in blotting applications by specifically visualizing the double-stranded complex, and to study the changes in DNA and analyze DNA content during apoptosis using flow cytometry. DAPI staining has also been shown to be a sensitive and specific detection method for mycoplasma. Spectral Data: Excitation: lambdaex 340 nm; Emission: lambdaem 488 nm (only DAPI). Excitation: lambdaex 360nm; Emission: lambdaem 460nm (DAPI-DNA complex).
Purity:
>95% (HPLC)
SMILES:
NC(C1=CC=C2C(NC(C3=CC=C(C(N)=N)C=C3)=C2)=C1)=N.Cl.Cl
Solubility Chemicals:
Soluble in water.
Transportation:
Non-hazardous
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) W.C. Russell, et al.; Nature 253, 461 (1975) | (2) I.W. Taylor & B.K. Milthorpe; J. Histochem. Cytochem. 28, 1224 (1980) | (3) F. Otto & K.C. Tsou; Stain Technol. 60, 7 (1985) | (4) C. Cubria, et al.; Comp. Biochem. Physiol. C. 105, 251 (1994) | (5) M.A. Hotz, et al.; Cytometry 15, 237 (1994) | (6) J. Kapuscinski; Biotech. Histochem. 70, 220 (1995) | (7) E. Buel & M. Schwartz; J. Forensic Sci. 40, 275 (1995) | (8) D. Zink, et al.; Methods: A Companion to Methods in Enzymology 29, 42 (2003) | (9) L. Rodgers; CSH Protoc. 2006, pdb.prot4438 (2006) | (10) B. Chazotte; CSH Protoc. 2011, pdb.prot5556 (2011) | (11) A. Biancardi, et al.; Phys. Chem. Chem. Phys. 15, 4596 (2013) | (12) M. Jez, et al.; Histochem. Cell Biol. 139, 195 (2013) | (13) G. Yahav, et al.; Cytometry A 89, 644 (2016)