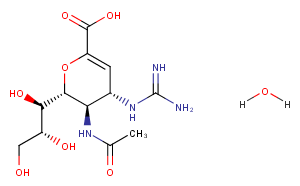
Zanamivir hydrate
Product Code:
TAR-T2529L
TAR-T2529L
Regulatory Status:
RUO
RUO
Shipping:
cool pack
cool pack
Storage:
-20℃
-20℃
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| TAR-T2529L-5mg | 5mg | £850.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT