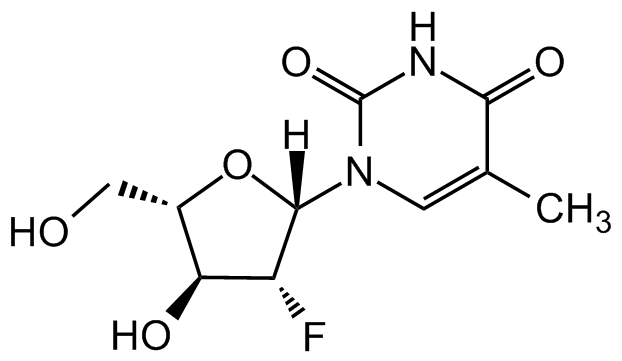
Clevudine
| Code | Size | Price |
|---|
| AG-CR1-3736-M005 | 5 mg | £40.00 |
Quantity:
| AG-CR1-3736-M010 | 10 mg | £55.00 |
Quantity:
| AG-CR1-3736-M050 | 50 mg | £100.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Images
Documents
Further Information
Alternate Names/Synonyms:
Levovir; L-FMAU; 2'-Fluoro-5-methyl-beta-L-arabinofuranosyl-uracil
Appearance:
White to off-white solid.
CAS:
163252-36-6
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C10H13FN2O5/c1-4-2-13(10(17)12-8(4)16)9-6(11)7(15)5(3-14)18-9/h2,5-7,9,14-15H,3H2,1H3,(H,12,16,17)/t5-,6+,7-,9-/m0/s1
InChiKey:
GBBJCSTXCAQSSJ-XQXXSGGOSA-N
Long Description:
Chemical. CAS: 163252-36-6. Formula: C10H13FN2O5. MW: 260.2. Clevudine is a synthetic pyrimidine nucleoside analog antiviral agent with activity against hepatitis B virus (HBV) and Epstein-Barr virus (EBV).
Intracellularly, clevudine is phosphorylated to its active metabolites, clevudine monophosphate and triphosphate. The triphosphate metabolite competes with thymidine for incorporation into viral DNA, thereby causing DNA chain termination and inhibiting the function of HBV DNA polymerase and reverse transcriptase, consequently blocking the viral replication. It also blocks the DNA supply of the virus into the nucleus, reducing the amount of cccDNA by 20-100 times.
Potentially useful for the treatment of the SARS-CoV-2 (COVID-19) infection, acting as a potent inhibitor of the RdRp protein.
MDL:
MFCD00935785
Molecular Formula:
C10H13FN2O5
Molecular Weight:
260.2
Package Type:
Vial
Product Description:
Clevudine is a synthetic pyrimidine nucleoside analog antiviral agent with activity against hepatitis B virus (HBV) and Epstein-Barr virus (EBV). Intracellularly, clevudine is phosphorylated to its active metabolites, clevudine monophosphate and triphosphate. The triphosphate metabolite competes with thymidine for incorporation into viral DNA, thereby causing DNA chain termination and inhibiting the function of HBV DNA polymerase and reverse transcriptase, consequently blocking the viral replication. It also blocks the DNA supply of the virus into the nucleus, reducing the amount of cccDNA by 20-100 times. Potentially useful for the treatment of the SARS-CoV-2 (COVID-19) infection, acting as a potent inhibitor of the RdRp protein.
Purity:
>98% (HPLC)
SMILES:
O[C@H]1[C@H](CO)O[C@]([H])(N2C=C(C)C(NC2=O)=O)[C@@H]1F
Solubility Chemicals:
Soluble in water (30mg/ml) or DMSO (20mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Use of 2'-fluoro-5-methyl-beta-L-arabinofuranosyluracil as a novel antiviral agent for hepatitis B virus and Epstein-Barr virus: C.K. Chu, et al.; Antimicrob. Agents Chemother. 39, 979 (1995) | Antiviral activity of clevudine [L-FMAU, (1-(2-fluoro-5-methyl-beta, L-arabinofuranosyl) uracil)] against woodchuck hepatitis virus replication and gene expression in chronically infected woodchucks (Marmota monax): S.F. Peek, et al.; Hepatology 33, 254 (2001) | Understanding the unique mechanism of L-FMAU (clevudine) against hepatitis B virus: molecular dynamics studies: Y. Chong & C.K. Chu; Bioorg. Med. Chem. Lett. 12, 3459 (2002) | Behavior of thymidylate kinase toward monophosphate metabolites and its role in the metabolism of 1-(2'-deoxy-2'-fluoro-beta-L-arabinofuranosyl)-5-methyluracil (Clevudine) and 2',3'-didehydro-2',3'-dideoxythymidine in cells: R. Hu, et al.; Antimicrob. Agents Chemother. 49, 2044 (2005) | Clevudine for the treatment of chronic hepatitis B virus infection: C.K. Hui & G.K. Lau; Expert Opin. Investig. Drugs 14, 1277 (2005) (Review) | Clevudine: a potent inhibitor of hepatitis B virus in vitro and in vivo: B.E. Korba, et al.; Expert Rev. Anti. Infect. Ther. 4, 549 (2006) (Review) | Clevudine is efficiently phosphorylated to the active triphosphate form in primary human hepatocytes: C. Niu, et al.; Antivir. Ther. 13, 263 (2008) | Evaluation of the in vitro anti-HBV activity of clevudine in combination with other nucleoside/nucleotide inhibitors: C. Niu, et al.; Antivir. Ther. 15, 401 (2010) | Noncompetitive inhibition of hepatitis B virus reverse transcriptase protein priming and DNA synthesis by the nucleoside analog clevudine: S.A. Jones, et al.; Antimicrob. Agents Chemother. 57, 4181 (2013) | Identification of Potent Drugs and Antiviral Agents for the Treatment of the SARS-CoV-2 Infection: N.R. Jena; ChemRxiv (Preprint) (2020)