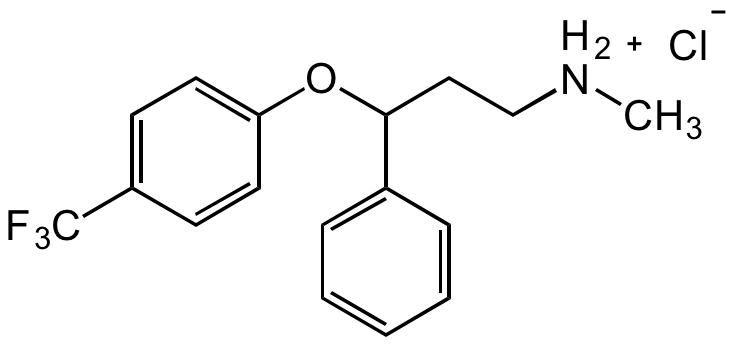
Fluoxetine hydrochloride
Product Code:
CDX-F0141
CDX-F0141
Antibody Isotype:
n/a
n/a
Antibody Clone:
n/a
n/a
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
+20°C
+20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-F0141-M010 | 10 mg | £35.00 |
Quantity:
| CDX-F0141-M050 | 50 mg | £72.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
(?)-N-Methyl-gamma-[4-(trifluoromethyl)phenoxy]benzenepropanamine hydrochloride; LY-110,140 hydrochloride; Prozac
Appearance:
White to off-white solid.
CAS:
56296-78-7
Class:
9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS05,GHS07,GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H302 + H332-H315-H318-H336-H373-H410
InChi:
InChI=1S/C17H18F3NO.ClH/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20;/h2-10,16,21H,11-12H2,1H3;1H
InChiKey:
GIYXAJPCNFJEHY-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 56296-78-7. Formula: C17H18F3NO . HCl. MW: 345.79. Fluoxetine is a cell-permeable selective serotonin reuptake inhibitor (SSRI), with preference for the serotonin transporter (Kd=0.81nM) over the norepinephrine transporter (Kd=240nM) and the dopamine transporter (Kd=3600nM). This drug works at presynaptic terminals where it prevents the reuptake of serotonin, resulting in the accumulation of serotonin in extracellular fluid at synapses. It functions as an antidepressant. Fluoxetine binds also to the human 5-HT transporter and is between 150- and 900- fold selective over 5-HT1A, 5-HT2A, H1, alpha1, alpha2-adrenergic, and muscarinic receptors. It has been shown to induce differentiation of neuronal precursors, enhancing neuronal characteristics. In addition, it was reported to modulate the proliferation of T-cells by increasing the Ca2+ influx and thereby influencing the activities of protein kinase A (PKA) and protein kinase C (PKC) and to regulate the phosphorylation of DARPP-32 and AMPA receptors.
MDL:
MFCD00214288
Molecular Formula:
C17H18F3NO . HCl
Molecular Weight:
345.79
Package Type:
Vial
PG:
III
Precautions:
P260-P280-P301 + P312 + P330-P305 + P351 + P338 + P310
Product Description:
Fluoxetine is a cell-permeable selective serotonin reuptake inhibitor (SSRI), with preference for the serotonin transporter (Kd=0.81nM) over the norepinephrine transporter (Kd=240nM) and the dopamine transporter (Kd=3600nM). This drug works at presynaptic terminals where it prevents the reuptake of serotonin, resulting in the accumulation of serotonin in extracellular fluid at synapses. It functions as an antidepressant. Fluoxetine binds also to the human 5-HT transporter and is between 150- and 900- fold selective over 5-HT1A, 5-HT2A, H1, alpha1, alpha2-adrenergic, and muscarinic receptors. It has been shown to induce differentiation of neuronal precursors, enhancing neuronal characteristics. In addition, it was reported to modulate the proliferation of T-cells by increasing the Ca2+ influx and thereby influencing the activities of protein kinase A (PKA) and protein kinase C (PKC) and to regulate the phosphorylation of DARPP-32 and AMPA receptors.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
C[NH2+]CCC(C1=CC=CC=C1)OC2=CC=C(C(F)(F)F)C=C2.[Cl-]
Solubility Chemicals:
Soluble in water (2mg/ml), DMSO (10mg/ml), DMF (10mg/ml) or ethanol (5mg/ml).
Source / Host:
Synthetic.
Transportation:
Excepted Quantity
UN Nummer:
3077
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
Documents
References
(1) P. Benfield, et al.; Drugs 32, 481 (1986) | (2) D.T. Wong, et al.; Life Sci. 57, 411 (1995) | (3) M. Tatsumi, et al.; Eur. J. Pharmacol 340, 249 (1997) | (4) S.G. Beck, et al.; J. Pharmacol. Exp. Ther. 281, 115 (1997) | (5) M.J. Owens, et al.; J. Pharmacol. Exp. Ther. 283, 1305 (1997) | (6) V.A. Edgar, et al.; Eur. J. Pharmacol. 372, 65 (1999) | (7) P. Bartholoma, et al.; Biochem. Pharmacol. 63, 1507 (2002) | (8) J.T. Bian, et al.; Eur. J. Pharmacol. 453, 159 (2002) | (9) A. Zhang, et al.; J. Med. Chem. 45, 1930 (2002) | (10) P. Svenningsson, et al.; PNAS 99, 3182 (2002) | (11) L. Perez-Caballero, et al.; Expert Opin. Drug Discov. 9, 567 (2014)