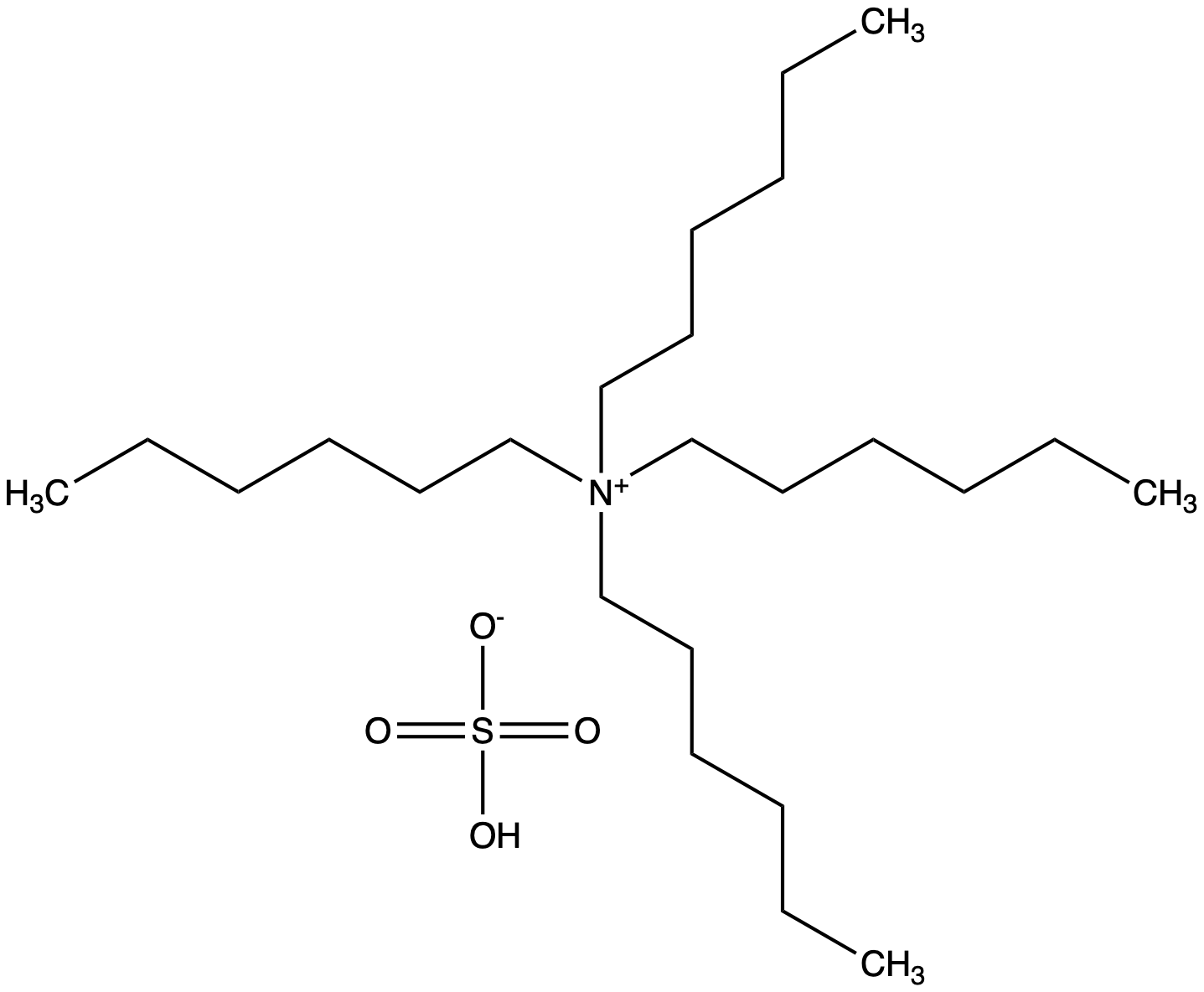
Tetrahexylammonium hydrogensulfate
Product Code:
CDX-T0610
CDX-T0610
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
Short term: +20°C, Long term: +20°C
Short term: +20°C, Long term: +20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-T0610-G005 | 5 g | £80.00 |
Quantity:
| CDX-T0610-G025 | 25 g | £242.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
Tetra-n-Hexylammonium hydrogen sulfate; THAHS
Appearance:
Colorless or white powder or crystals.
CAS:
32503-34-7
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Keep under inert gas. Very hygroscopic.
Hazards:
H315 - H319 - H335
InChi:
InChi=1S/C24H52N.H2O4S/c1-5-9-13-17-21-25(22-18-14-10-6-2,23-19-15-11-7-3)24-20-16-12-8-4;1-5(2,3)4/h5-24H2,1-4H3;(H2,1,2,3,4)/q+1;/p-1
InChiKey:
RULHPTADXJPDSN-UHFFFAOYSA-M
Long Description:
Chemical. CAS: 32503-34-7. Formula: C24H53NO4S. MW: 451.75. Tetrahexylammonium hydrogensulfate (THAHS) is a quaternary ammonium salt used in various chemical and biochemical applications. It consists of a tetracationic ammonium ion (C6H13)4N+ and a hydrogen sulfate anion (HSO4-). THAHS is used as a phase-transfer catalyst (PCT) and can facilitate various chemical reactions by aiding in the transfer of reactants between immiscible phases. Phase-transfer catalysis involves the transfer of reactants from one phase (usually an aqueous phase) to another phase (usually an organic phase) to facilitate a chemical reaction. THAHS can be employed in organic synthesis, particularly in reactions that involve the transfer of anions or reagents between organic and aqueous phases. Some common applications include, quaternization reactions, nucleophilic substitution reactions or halogenation reactions. It can also be used in processes that involve the extraction or separation of specific ions or compounds from one phase to another.
MDL:
MFCD00037675
Molecular Formula:
C24H53NO4S
Molecular Weight:
451.75
Package Type:
Vial
Precautions:
P302 + P352 - P305 + P351 + P338
Product Description:
Tetrahexylammonium hydrogensulfate (THAHS) is a quaternary ammonium salt used in various chemical and biochemical applications. It consists of a tetracationic ammonium ion (C6H13)4N+ and a hydrogen sulfate anion (HSO4-). THAHS is used as a phase-transfer catalyst (PCT) and can facilitate various chemical reactions by aiding in the transfer of reactants between immiscible phases. Phase-transfer catalysis involves the transfer of reactants from one phase (usually an aqueous phase) to another phase (usually an organic phase) to facilitate a chemical reaction. THAHS can be employed in organic synthesis, particularly in reactions that involve the transfer of anions or reagents between organic and aqueous phases. Some common applications include, quaternization reactions, nucleophilic substitution reactions or halogenation reactions. It can also be used in processes that involve the extraction or separation of specific ions or compounds from one phase to another.
Purity:
>98% (Titr.)
Signal Word:
Warning
SMILES:
OS([O-])(=O)=O.CCCCCC[N+](CCCCCC)(CCCCCC)CCCCCC
Solubility Chemicals:
Soluble in methanol or DMSO.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
Documents
References
(1) R. Bar, et al.; J. Mol. Catal. 16, 175 (1982) | (2) D. Feldmann & M. Rabinovitz; J. Org. Chem. 53, 3779 (1988) | (3) L.G Sayers, et al.; Biochem. Soc. Trans. 21, 105S (1993) | (4) T.S. Straub; Tetrahedr. Lett. 36, 663 (1995) | (5) S. Amaratunga & H. Alper; J. Organometall. Chem. 488, 25 (1995) | (6) L.J. Csanyi & K. Jaky; Phys. Chem. Chem. Phys. 3, 2018 (2001) | (7) R.J. Fox & J. Qiu; Org. Process Res. Dev. 24, 235 (2020)